Answer: Protein kinases are enzymes that alter synaptic function by catalyzing chemical reactions in the brain, which can lead to plasticity such as long term potentiation (LTP) or long term depression (LTD).
Article written by Khayla Black
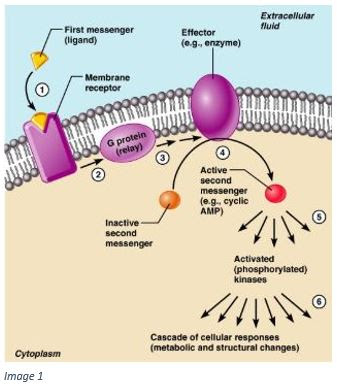
Protein kinases are enzymes which play a critical role in the process by which chemical signals are converted into biological responses in target cells. Upon activation, these enzymes catalyze specific reactions in a process known as phosphorylation, where a phosphate group is transferred from ATP to cellular proteins. This process is essential for many neurological processes but is especially significant for understanding the cellular basis of learning and memory.
Protein kinases are activated by chemicals known as second messengers. To understand this activation, we can think of a signaling cascade initiated with the activation of a G-protein. A chemical signal, such as a neurotransmitter, binds to a membrane receptor and activates a G-protein, which serves as the first messenger. The G-protein activates an effector (such as adenylyl cyclase) which catalyzes the reaction which then activates a second messenger. For example, adenylyl cyclase catalyzes the reaction, converting ATP to cyclic AMP. The second messenger will then stimulate protein kinases, which catalyze phosphorylation.
Phosphorylation is an essential step in the biochemical process of converting chemical signals into biological responses. Many extracellular signals regulate phosphorylation in order to control the activity of target proteins. The process of adding a phosphate group to a protein generally results in a conformational change in the protein, which significantly impacts its function.
Synaptic transmission is known to be regulated by protein phosphorylation, and the action of Calcium/Calmodulin-dependent kinase II (CaMKII) allows protein phosphorylation to become a permanent change. For LTP to occur, both influx of calcium and activation of CaMKII are required. The role of CaMKII is to catalyze phosphorylation of proteins in response to an influx in Ca2+ Calmodulin (a calcium binding messenger protein). One thing that is special about CaMKII is that it continues to phosphorylate proteins even when it is not being activated by a second messenger. This is due to the structure of the enzyme. Image 2 displays the structure, showing both the catalytic and the regulatory region. Envision that the enzyme opens like a door, and the point in which the regulatory and catalytic regions connect serves as the hinge. When a second messenger is present, the enzyme will “open”, exposing the catalytic region, which is responsible for phosphorylation. One of the key features of CaMKII is that it is an autophosphorylating compound, meaning each of the subunits within the enzyme can phosphorylate the neighboring one. This allows a contribution to synaptic modification for hours.
In addition to CaMKII, there are other types of protein kinases which are heavily involved in memory, namely Protein kinase C, or PKC. PKC is another family of kinase enzymes which play a significant role in LTD in the cerebellum. Three signals are essential for the initiation of cerebellar LTD: a rise in calcium due to climbing fiber activation, rise in sodium due to AMPA receptor activation, and activation of PKC due to metabotropic receptor activation. When the parallel fibers are activated in LTD, they release glutamate, which can act on the metabotropic glutamate receptors postsynaptic to the parallel fiber. The metabotropic glutamate receptor activates a G-protein which activates the enzyme phospholipase C. This leads to the production of diacylglycerol which activates PKC. PKC is believed to then play a role in the mechanism which results in a decrease in AMPA receptors in the postsynaptic membrane.
Though there is certainly much more to be discovered regarding the activity of protein kinases, it is evident that that, by phosphorylating proteins, these enzymes play a profound role in regulating synaptic functions. Their role in LTP and LTD make them essential components in our current models for learning and memory.