Written by Khayla Black
Answer: Synaptic vesicles filled with neurotransmitter undergo a process called ‘synaptic vesicle exocytosis’ which released neurotransmitter into the synaptic cleft.
Synapses are extraordinarily important for neuronal communication; we can think of each synapse as a phone and the neurotransmitters that pass between them as individual text messages. In order to transfer information (in the form of chemical messages - neurotransmitters), the presynaptic and postsynaptic neurons must come into contact. When these neurons come together, they do not actually touch; however, they do come extremely close and form a space between the neurons known as the synaptic cleft. In order to deliver its message, the presynaptic neuron has to get its chemical messengers to the postsynaptic neuron. To do this, neurotransmitters are released from the presynaptic neuron, and they diffuse across the synaptic cleft. Eventually, they reach the postsynaptic neuron and bind to receptors there where they can initiate chemical changes.
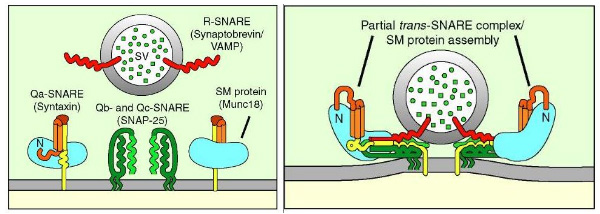
The process by which neurotransmitters are released from the presynaptic neuron into the synaptic cleft is known as ‘synaptic vesicle exocytosis’ and is extremely complex. Synaptic vesicle exocytosis is mediated by a SNARE/SM protein cycle in which the ATPase NSF acts with a group of proteins known as SNAP’s to provide the energy necessary for the process to occur. To understand this process fully, we need to consider the presynaptic neuron on a more detailed level. The figure to the left shows components of the presynaptic neuron including the neuronal membrane, a synaptic vesicle, and some proteins. The first protein, synaptobrevin, is attached to the vesicular membrane, and on the neuronal membrane, we can see the second protein, SNAP-25 along with a complex. This complex consists of two proteins - Munc18 and syntaxin-1.
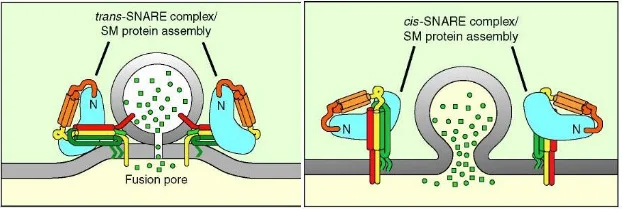
Recall that synaptobrevin is located on the vesicular membrane, while syntaxin and SNAP-25 are located on the neuronal membrane; for this reason, we call synaptobrevin a V-SNARE (V for vesicular) and SNAP-25 and syntaxin-1 T-SNAREs (T for target). Once fusion is initiated (a process which will be discussed shortly), these three proteins “zipper together” to form what is known as the SNARE complex, shown in the image to the above. The SNARE complex works to tug on the membranes and form an opening in both the vesicle and the neuronal membrane. The opening in the membrane allows the vesicle to collapse into the membrane; the membranes fuse into one, neurotransmitters flow out of the vesicle, and the SNARE proteins can then “let go” of the vesicular membrane. This allows the complex to reside fully on the neuronal membrane, and we say this represents the trans-SNARE complex converting into a cis-SNARE complex, as shown below.
Once fusion is complete and neurotransmitter has been released, the cis-SNARE complexes still reside on the membrane. In order to prepare the presynaptic neuron for another round of fusion, NSF and SNAPs come in and dissociate the complexes. This dissociation frees the SNARE proteins up and provides the energy needed to facilitate another round of fusion.
We can take a step back and consider how this entire mechanism of exocytosis is initiated. To understand this, we need to bring two more proteins into the picture, synaptotagmin and complexin. At any point in time, there exists a collection of vesicles docked at the membrane ready for release; however, there must be a mechanism in place to hold them there. This is where complexin comes in. Complexin acts as a clamp and binds to the SNARE complex, preventing full zippering of the SNARE proteins. Essentially, it allows the trans-SNARE complex to form and “wait” at the membrane for the signal. At low levels of calcium, this clamp can function particularly well. However, when calcium levels rise, synaptotagmin (a calcium sensor) is activated, complexin is released, and the vesicles are allowed to fuse.
In addition to synaptotagmin and complexin, there is another component which can be examined in much greater detail, namely, Munc18. Recall previously when it was mentioned that there is a complex on the target membrane initially composed of Munc18 and syntaxin. Munc18 is an example of a protein known as an SM protein. SM proteins function as clasps that organize SNARE complexes and assist them in “zippering together”. The figure to the left demonstrates an SM protein clasping the SNAREpin (aka SNARE complex). SM proteins fold into an arch formation, allowing them to bind individually to syntaxin. They become anchored together, and SM acts as a catalyst for SNAREs which are, in and of themselves, catalysts for fusion.
Although a great deal is known about the processes surrounding synaptic transmission, there are many processes and mechanisms that remain to be elucidated. Hopefully future breakthroughs in neurophysiology will improve our understanding of these incredibly complex processes and provide insight into neurological disorders of synaptic transmission.